What Are Schedule 3 Drugs? Meaning, List and Why It’s in the News
What “Schedule 3” Actually Means
In the United States, not all drugs are treated the same under federal law. The government uses a system called “drug scheduling” to rank substances from Schedule I (the most tightly restricted) to Schedule V (the least restricted). Schedule 3 drugs sit right in the middle of that scale.
Schedule 3 – often written as “Schedule III” – covers drugs that do have accepted medical uses but still carry a risk of abuse and dependence. They are considered less dangerous than Schedule I and Schedule II substances, yet more tightly controlled than everyday prescription medicines. You usually need a valid prescription, careful monitoring and specific paperwork if you’re a doctor, pharmacist or researcher dealing with them.
Legal Definition of Schedule 3 Drugs
Under US federal law, a drug is typically placed in Schedule 3 if it meets three basic conditions:
- Its potential for abuse is lower than Schedule I and Schedule II drugs, but not zero.
- It has a currently accepted medical use in treatment in the United States.
- Abuse of the drug may lead to moderate or low physical dependence, or a higher level of psychological dependence, but generally less severe than heroin‑ or fentanyl‑level addiction.
That mix is why Schedule 3 substances are sometimes called “middle‑tier” controlled drugs. They can be very helpful when used correctly, yet still risky enough that the government wants them tracked, prescribed and dispensed with extra care.
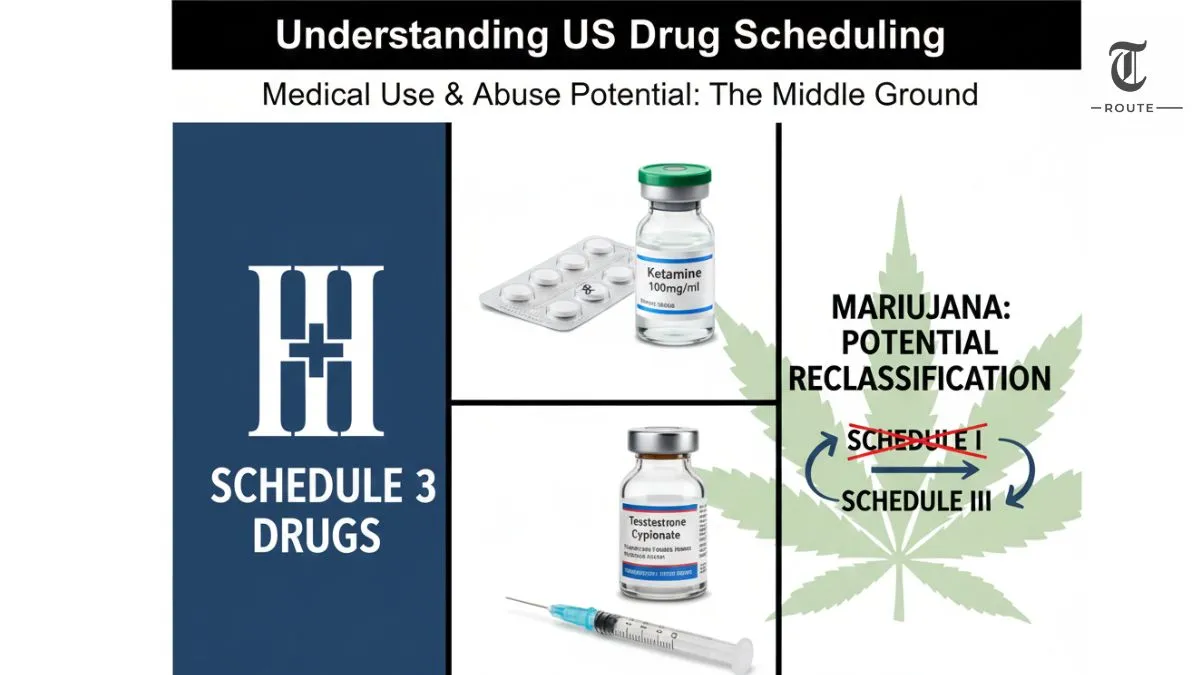
Common Examples of Schedule 3 Drugs
Most people are surprised to learn how familiar some Schedule 3 drugs actually are. This category combines pain medicines, hormone drugs and certain anesthetics. Well‑known examples include:
- Products containing codeine at lower doses – for example, some formulations similar to Tylenol with codeine that contain less than a set amount of codeine per dose.
- Ketamine – an anesthetic used in surgery and pain management, also sometimes misused recreationally.
- Anabolic steroids – synthetic hormones used medically for certain conditions but often abused for bodybuilding and performance enhancement.
- Testosterone and some other hormone therapies – prescribed for medical reasons but with abuse potential in sports and fitness circles.
The exact official list is long and technical, but most fall into these broad buckets: pain relief, anesthesia, and hormone or steroid‑related treatments. All of them require a prescription and are closely regulated at the pharmacy level.
Why Schedule 3 Is Suddenly in the News
Schedule 3 drugs have been part of US law for decades, but the phrase is trending again for one key reason: marijuana. After years of debate, the US government has been moving to reclassify cannabis from Schedule I (the strictest category) to Schedule III under federal law.
If that change fully takes effect, it will not make marijuana completely “legal” at the federal level, but it will acknowledge medical use and place it in the same schedule as drugs like ketamine, certain codeine products and anabolic steroids. That shift could make research easier, change how businesses are taxed and reshape how federal authorities treat cannabis, even as state laws continue to vary.
How Schedule 3 Differs from Other Schedules
To understand Schedule 3, it helps to see it in context:
- Schedule I: Highest restriction. No accepted medical use at the federal level and a high potential for abuse. Examples historically included heroin and, until rescheduling, marijuana.
- Schedule II: Accepted medical use but very high abuse and dependence risk. Examples include oxycodone, fentanyl and some ADHD medications.
- Schedule III: Accepted medical use, moderate abuse risk, and moderate or low physical dependence compared to Schedule II. This is the “middle ground” tier.
- Schedule IV and V: Lower abuse potential, commonly prescribed medications like certain anti‑anxiety drugs, sleep aids and cough syrups.
For patients, that usually means Schedule 3 medicines are easier to access than the most tightly controlled narcotics but still come with stricter rules than typical prescriptions. For doctors, they involve DEA registration, recordkeeping and limits on refills and dispensing.
Why Schedule 3 Matters for Patients and Policy
For patients, Schedule 3 classification can affect how easily a treatment can be prescribed, how often refills are allowed, and how much scrutiny comes with long‑term use. A drug in this category might offer real relief, but it won’t be treated like a simple over‑the‑counter remedy.
For policymakers, Schedule 3 is a balancing tool: it signals that a substance has legitimate medical value, but it also keeps the door open for enforcement if the drug is diverted, over‑prescribed or trafficked. The marijuana debate shows how powerful that label can be — moving a substance into Schedule III can change research rules, tax treatment and political arguments, all without fully removing it from the controlled‑substance system.