Schedule 1 vs Schedule 2 vs Schedule 3 Drugs: Key Differences Explained
Why US Drug Schedules Matter
In the United States, not every drug is treated the same. Federal law uses a “schedule” system to classify controlled substances from Schedule I (the strictest) to Schedule V (the least restricted. The schedule a drug is placed in affects everything: how it can be prescribed, how tightly it is tracked, and what penalties apply if it’s abused or trafficked.
The three most talked‑about categories are Schedule 1, Schedule 2 and Schedule 3. They all involve substances with abuse potential, but the law draws sharp lines between them. Below is a clear, side‑by‑side breakdown—including a comparison table you can skim in seconds.
Schedule 1 vs Schedule 2 vs Schedule 3: Quick Comparison
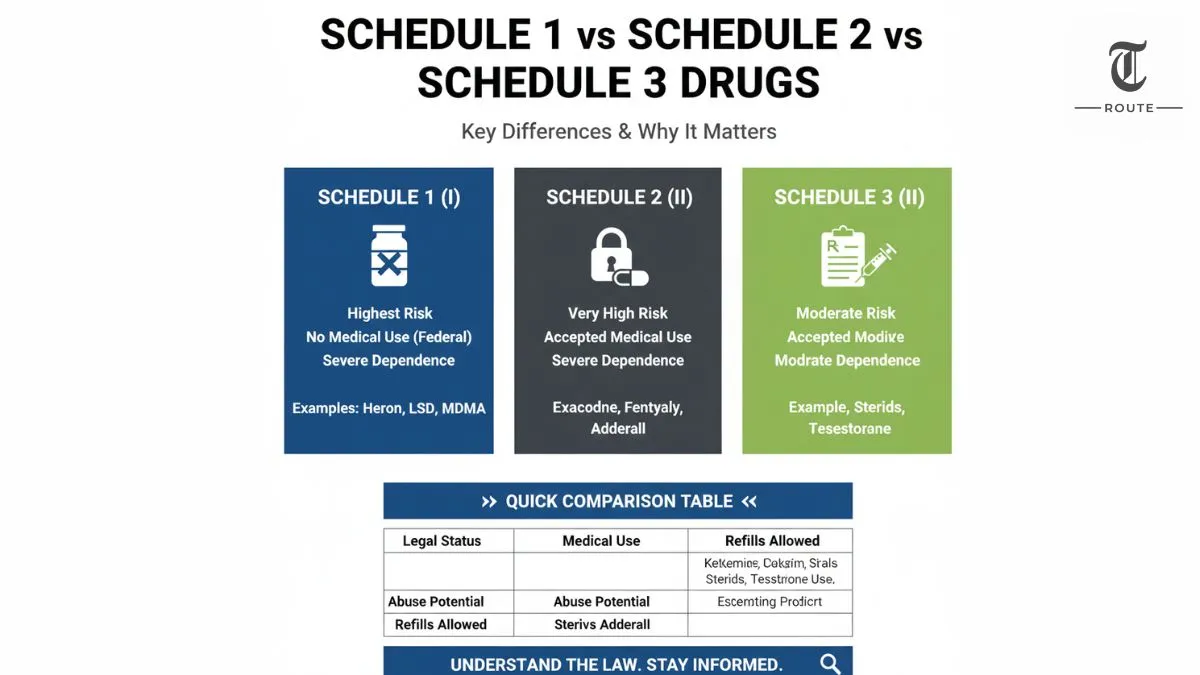
| Feature | Schedule 1 (I) | Schedule 2 (II) | Schedule 3 (III) |
|---|---|---|---|
| Legal status under federal law | Illegal to prescribe; no accepted medical use | Legal with strict prescription rules | Legal with controlled prescription rules |
| Accepted medical use | None (federally) | Yes, but highly restricted | Yes, recognized medical use |
| Abuse potential | Highest | Very high | Moderate |
| Risk of dependence | Severe psychological and/or physical dependence | Severe psychological or physical dependence | Moderate or low physical dependence; high psychological dependence possible |
| Typical examples | Heroin, LSD, MDMA (ecstasy), peyote | Oxycodone, fentanyl, morphine, amphetamine (Adderall), cocaine | Ketamine, anabolic steroids, testosterone products, certain codeine combinations |
| Prescription allowed? | No | Yes, no refills without a new prescription | Yes, with limited refills and monitoring |
| Common use context | Illicit / recreational only (under federal law) | Severe pain, ADHD, anesthesia, with high diversion risk | Pain relief, anesthesia, hormone therapy, with abuse risks |
Schedule 1 Drugs: Highest Control, No Accepted Medical Use
Schedule 1 drugs are considered the most restricted category under US federal law. The government defines them as substances with a high potential for abuse and no currently accepted medical use in treatment at the federal level. Because of that, they cannot be prescribed by doctors, even if some states have their own laws that say otherwise.
Classic examples include heroin, LSD, MDMA (ecstasy), and certain hallucinogens. Research is possible, but it requires special federal approval and is heavily regulated. In practice, these drugs are treated as illegal across the board in federal eyes, which is why Schedule I carries the toughest legal consequences.
Schedule 2 Drugs: Powerful Medicines With Very High Abuse Risk
Schedule 2 drugs sit one step below Schedule 1 in terms of strictness, but they are still considered extremely risky. The key difference is that Schedule 2 substances do have accepted medical uses. They can be prescribed for specific conditions, but under tight controls and with significant legal scrutiny.
This category includes strong opioid painkillers like oxycodone and fentanyl, stimulant medications such as amphetamine (Adderall) and methylphenidate (Ritalin), and even cocaine when used in certain medical settings. Abuse potential is very high, and misuse can lead to severe psychological or physical dependence. Prescriptions are usually limited, refills often require a fresh script, and pharmacists must follow strict DEA rules when dispensing these medications.
Schedule 3 Drugs: The “Middle Ground” Category
Schedule 3 drugs (Schedule III) occupy the middle tier. They still carry abuse and dependence risks, but those risks are considered lower than Schedule 1 and Schedule 2 substances. At the same time, they clearly have accepted medical uses, and many are prescribed routinely under supervision.
Common examples include ketamine, certain combination products with lower doses of codeine, anabolic steroids and testosterone therapies. These drugs can lead to moderate or low physical dependence and higher psychological dependence, especially when misused over time. They are available by prescription, often with limited refills and recordkeeping requirements. For a deep dive into this category, see your dedicated guide on schedule 3 drug meaning (internal link to your Schedule 3 article).
Abuse Potential: How the Schedules Stack Up
In simple terms, the schedules can be visualized as a gradient of risk:
- Schedule 1: Very high abuse potential, no accepted medical use.
- Schedule 2: Very high abuse potential, but important medical uses.
- Schedule 3: Moderate abuse potential, established medical uses.
That gradient matters in court, in clinics and in pharmacies. The higher the schedule number, the easier it generally is to prescribe and refill; the lower the schedule number, the more serious the legal and regulatory environment around the drug.
Medical Use: Where the Line Is Drawn
The second major factor is accepted medical use under federal law:
- Schedule 1: Officially, none. Even if states allow medical or recreational use (for example, with cannabis before rescheduling), federal law still treats these substances as having no accepted medical role.
- Schedule 2: Clear medical uses, but they are powerful enough that doctors must weigh benefits against very serious risks of addiction and diversion.
- Schedule 3: Recognized medical uses with a better safety margin than Schedule 2, but still enough risk that the government keeps them in the controlled‑substance system.
This is why placement on the schedule can be politically explosive: moving a drug down the ladder (for example, from Schedule I to Schedule III) signals that the federal government acknowledges medical value and is willing to relax certain barriers while still keeping monitoring in place.
Why This Comparison Matters Now
Debates around opioids, stimulants and cannabis have pushed the details of drug scheduling out of legal textbooks and into everyday news. Whether it’s an opioid crisis story, a case about steroid abuse in sports, or discussion of marijuana being moved to Schedule III, the same core questions keep coming up: How dangerous is this substance? How useful is it in medicine? And where should the law draw the line?
Understanding the differences between Schedule 1, Schedule 2 and Schedule 3 drugs gives you a clearer lens on those debates. It explains why some medications require special triplicate scripts, why certain drugs attract harsher penalties than others, and why rescheduling decisions can reshape entire industries overnight.